

Cannabis Research
First mover in cannabis research
At Landsteiner Scientific®, together with the main academic institutions in the country, we have explored into cannabis research for the development of products with CBD or Cannabidiol, the most important cannabinoid in the plant with high therapeutic potential without psychoactive effect. Find out more about our innovative developments and current research protocols.CannSpheres, our proprietary delivery system
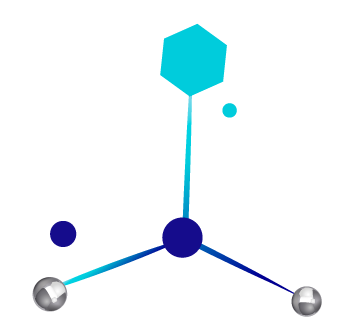
CannSpheres, patent Landsteiner Scientific®
Cannspheres are liposome systems, which are nanoparticles and microparticles vesicles formed by one or more lipid bilayer membranes in which the active ingredient is encapsulated, which increases the bioavailability of the active ingredients.
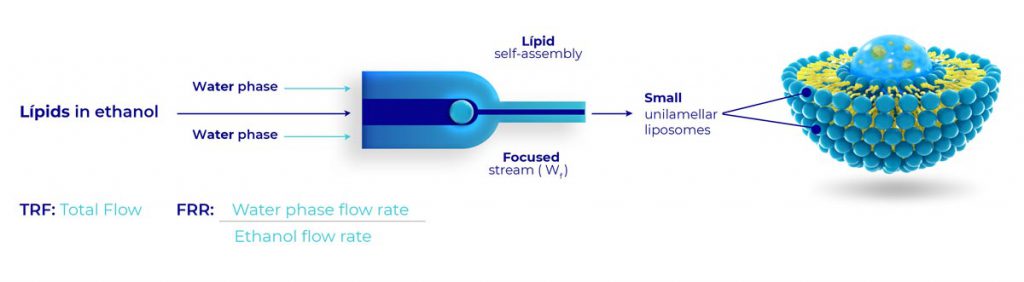
The delivery system is proprietary and facilitates increased bioavailability. Specified release depending on the designed particle size < 100 nm particles are used for topicals and ophthalmic applications. Larger particles for gastro-intestinal absorption.

This is a revolutionary delivery system for Cannabinoids for faster absorption with utility across a wide range of applications including drinking water and enteral medications.
The patent application will be submitted in Japan, US, México, and EU before long.
Development of medical cannabis product
- Library of naturally occurring cannabinoids
- Lost of them are unknown in terms of which biological activity they have
- The “entourage effect” points to several having large activities for different conditions
- After the preliminary screening assays directed to the new designed target, a series of compounds remain as candidates for development or “Hits”
- Furthermore, strict screening of the Hits leads to compounds with the best pharmacological properties desired for DNA.
- Oral Bioavailability, Blood Brain Barrier crossing, specific activity, etc.
- The compound(s) to be developed in the new medicine are optimized with in vivo and in vitro studies.
Research Protocols
IBS (inflammatory Bowel Syndrome) Migraine / headache
Protocol Name: Biological effects of Cannabidiol and clinical response in Mexican patients with IBD
Doctor: Dra. Teresita Navarrete Cruces
Research Institution: General Hospital of Mexico
Collaborative Hospital: General Hospital of Mexico
Number of patients: 50 (2 groups of 25 each one included Placebo)
Migraine/headache
Protocol Name: Effect of THC/CBD as a coadjuvant in the treatment of patients with migraine in patients with chronic migraine and exacerbation
Doctor: Dr. Adolfo Leyva Rendón
Research Institution: National Institute of Neurology and Neurosurgery
Collaborative Hospital: National Institute of Neurology and Neurosurgery
Number of patients: 60 (2 groups of 30 each one included Placebo)
Gastric Cancer
Protocol Name: Effect of THC/CBD as a coadjuvant in the treatment of patients with gastric cancer
Doctor: Dra. Erika Rufz
Research Institution: National Institute of Cancer
Collaborative Hospital: Research unit at BioMedica in Cancer
Number of patients: 32 (2 groups of 16 each one)
Fibromyalgia Syndrome
Protocol Name: Study of cannabinoids (THC/CBD) in pain: Fibromyalgia Syndrome
Doctor: Dra. Luz Adriana Templos
Research Institution: General Hospital “Dr. Manuel GEA González’
Collaborative Hospital: General Hospital “Dr. Manuel GEA González”
Glaucoma
Protocol Name: Study of cannabinoids (THC/CBD) in Glaucoma Nausea and Vomiting Control
Control de náuseas y vómitos
Protocol Name: Multicenter randomized open study to evaluate the efficacy and safety of different cannabinoids as adjuvants in the treatment of Nausea and Vomiting for 70 weeks
Doctor: Dra. Erika Hernandez Guevara
Research Institution: National Autonomous University of Mexico
Collaborative Hospital: Regional Hospital of high specialty of lxtapaluca
Number of patients: 300 (70 groups of 30 each one)
Dysmenorrhea
Protocol Name: Study of cannabinoids (THC/CBD) in pain: Premenstrual Syndrome
Doctor: Dra. Elsa Diaz Lopez
Research Institution: Hospital Ángeles Mocel
Collaborative HospitaI: Hospital Ángeles Mocel
Psoriasis
Protocol Name: Study of cannabinoids (THC/CBD) in Psoriasis
Doctor: Dr. Fernando Blancas
Palliative Oncology
Protocol Name: Pilot study about the treatment effect of cannabinoids (THC/CBD) in the control of pain and opioids requirements in palliative oncology patients
Doctor: Dra. Silvia Allende
Research Institution: INCAN
Collaborative Hospital: Research unit at Bio Medica in Cancer
Number of patients: 80 (3 groups of 20 each one + 20 Placebo)
Trigeminal Neuralgia
Protocol Name: Multicenter randomized open study to evaluate the efficacy and safety of different cannabinoids as adjuvant in the treatment of Trigeminal Neuralgia for 10 weeks
Doctor: Dra. Erika Hernandez Guevara
Research Institution: UNAM
Collaborative Hospital: Regional Hospital of high specialty of lxtapaluca
Number of patients: 300 (70 groups of 30 each one)
Osteoarthritis
Protocol Name: Multicenter randomized open study to evaluate the efficacy and safety of different cannabinoids as adjuvants in the treatment of Osteoarthritis for 70 weeks
Doctor: Dra. Erika Hernandez Guevara
Research Institution: UNAM
Collaborative Hospital: Regional Hospital of high specialty of lxtapaluca
Number of patients: 300 (70 groups of 30 each one)
Chronic Low Back Pain
Protocol Name: Multicenter randomized open study to evaluate the efficacy and safety of different cannabinoids as adjuvants in the treatment of Chronic Low Back Pain for 10 weeks
Doctor: Dra. Erika Hernandez Guevara
Research Institution: UNAM
Collaborative Hospital: Regional Hospital of high specialty of lxtapaluca
Number of patients: 300 (70 groups of 30 each one)
Pain Sports
Protocol Name: Effect of CBD as a coadjuvant in the treatment amateur sportsmen with pain
Doctor: Dr. Jose Meynardo Cedillo Ubaldo
Research Institution: Angeles Hospital
Collaborative Hospital: Angeles Coapa Hospital
Number of patients: 60 (2 groups of 30 each one included Placebo)
Healthy Dogs
Protocol Name: Determination of Cannabidiol (CBD) pharmacokinetics parameters in healthy dogs.
Research Institution: UAEM Autonomous University of Morelos State
Collaborative Hospital: Faculty of veterinary medicine and zootechnics
Number of patients: 24 (3 groups of 8 each one) & 10 (2 groups of 5 each one)

